Nutrient Cycles Biogeochemical Interactions


Nutrient Cycles Outcomes
-
List examples of different nutrients.
-
Provide the sinks and general cycling of water, carbon, and nitrogen.
-
Explain how acid rain is formed and describe its effects.
Nutrients are the building blocks of larger molecules.
Nutrients refer to elements and simple molecules (-chemical) that travel through living (bio-) and nonliving (geo-) parts of Earth.
Biogeochemical cycles refer to the movement of nutrients and their molecular forms through organisms and non-living materials.
We primarily hear about nutrients related to food content, We need to regularly ingest a variety of nutrients for our cells to function.
Examples of nutrient (biogeochemical) cycles.
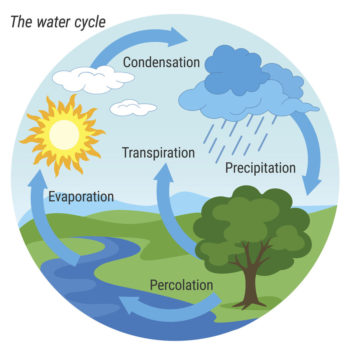
The water cycle is the most familiar cycle since we are strongly impacted by precipitation (rain, fog, snow, sleet). Less that 1% of the Earth\’s water is found in the atmosphere, but that small aount has a significant impact on life.
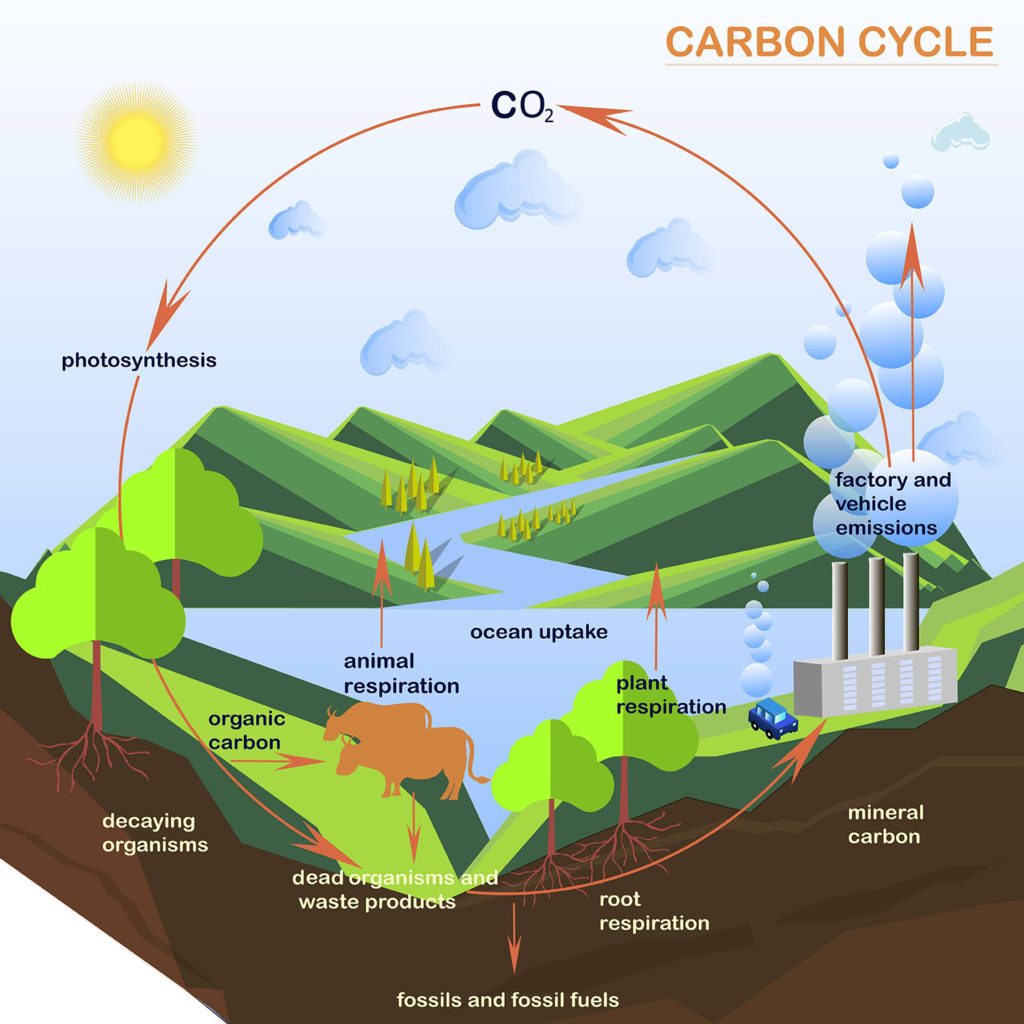
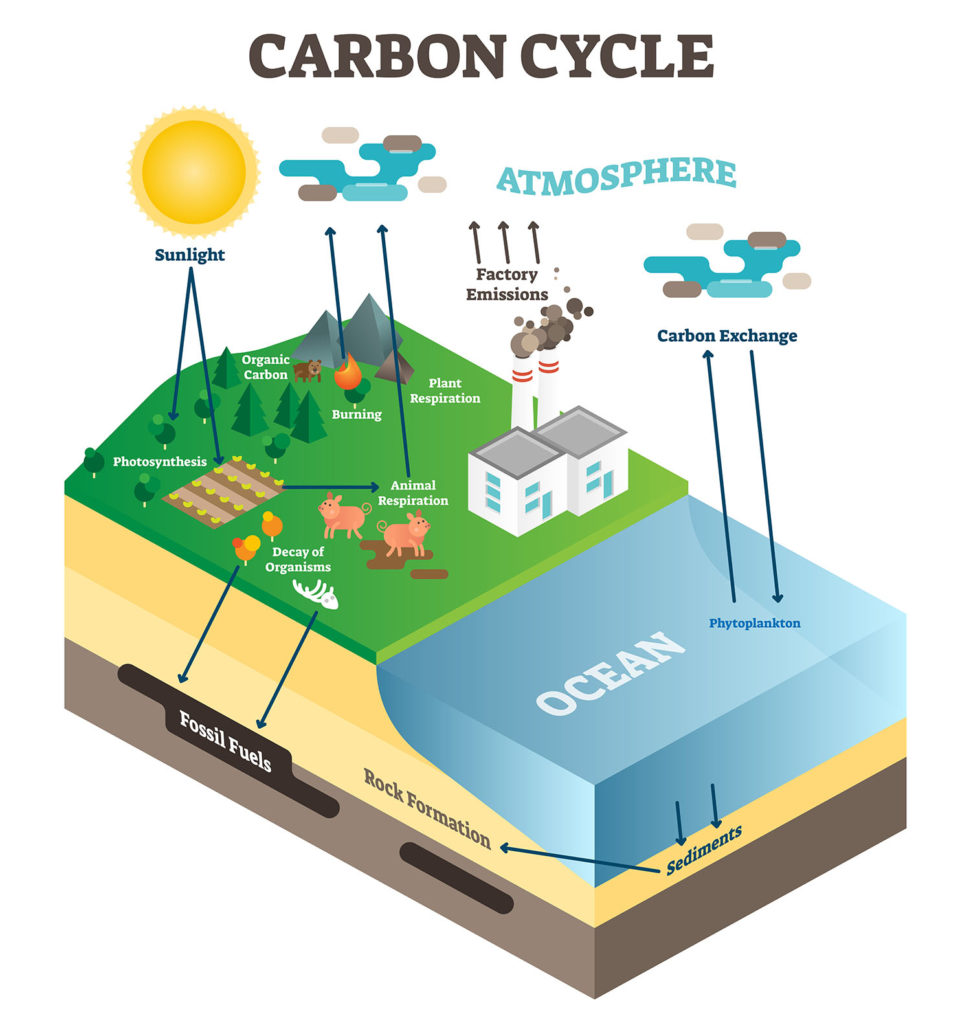
Carbon, along with oxygen and water, is a key element in photosynthesis and respiration. When you exhale, you are releasing carbon dioxide into the atmosphere, which producers can utilize for photosynthesis.
The carbon cycle gets a lot of media attention because carbon-containing molecules like carbon dioxide and methane retain heat in the atmosphere.
Human activities have increased these gases over natural sources (volcanic eruptions, forest fires, respiration). More on this in the atmosphere and climate resource.
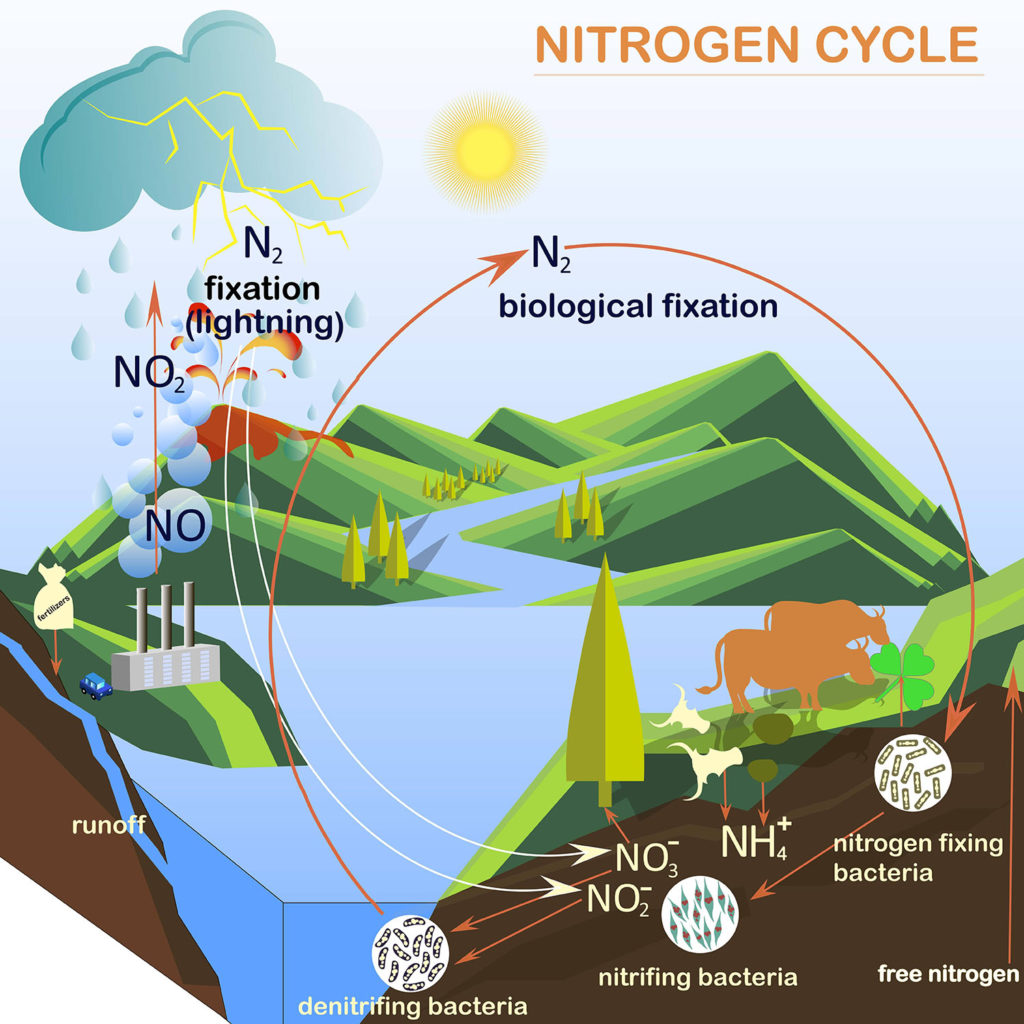
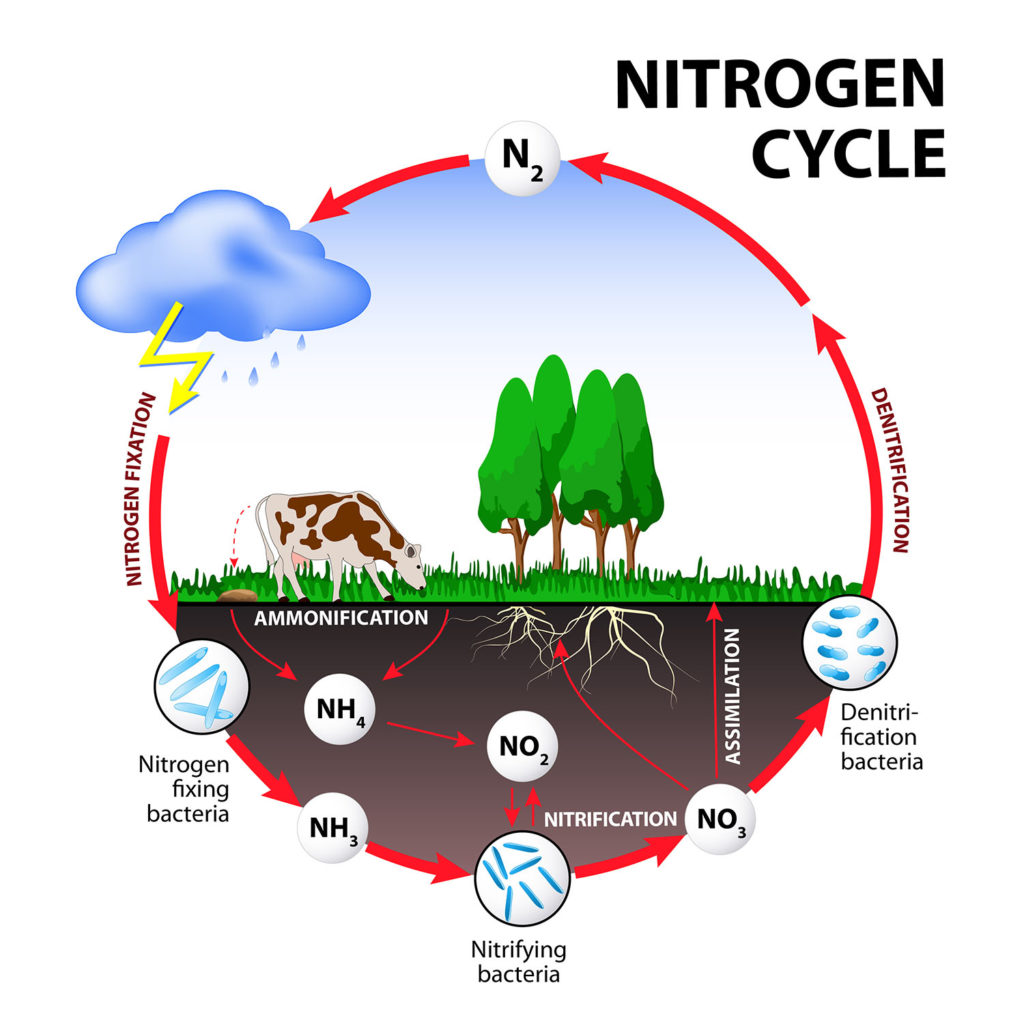
With every breath we take in more nitrogen gas than oxygen, but we can\’t use it. We have to get the nitrogen from the food we eat. The food we eat ultimately has to get nitrogen from the soil.
Bacteria play a significant role in making atmospheric nitrogen available to organisms growing in the soil.
Gases can be released into the atmosphere through volcanic eruptions, forest fires, grassland fires, and even asteroid impacts.
In the past two centuries, humans have significantly increased emissions into the atmosphere.
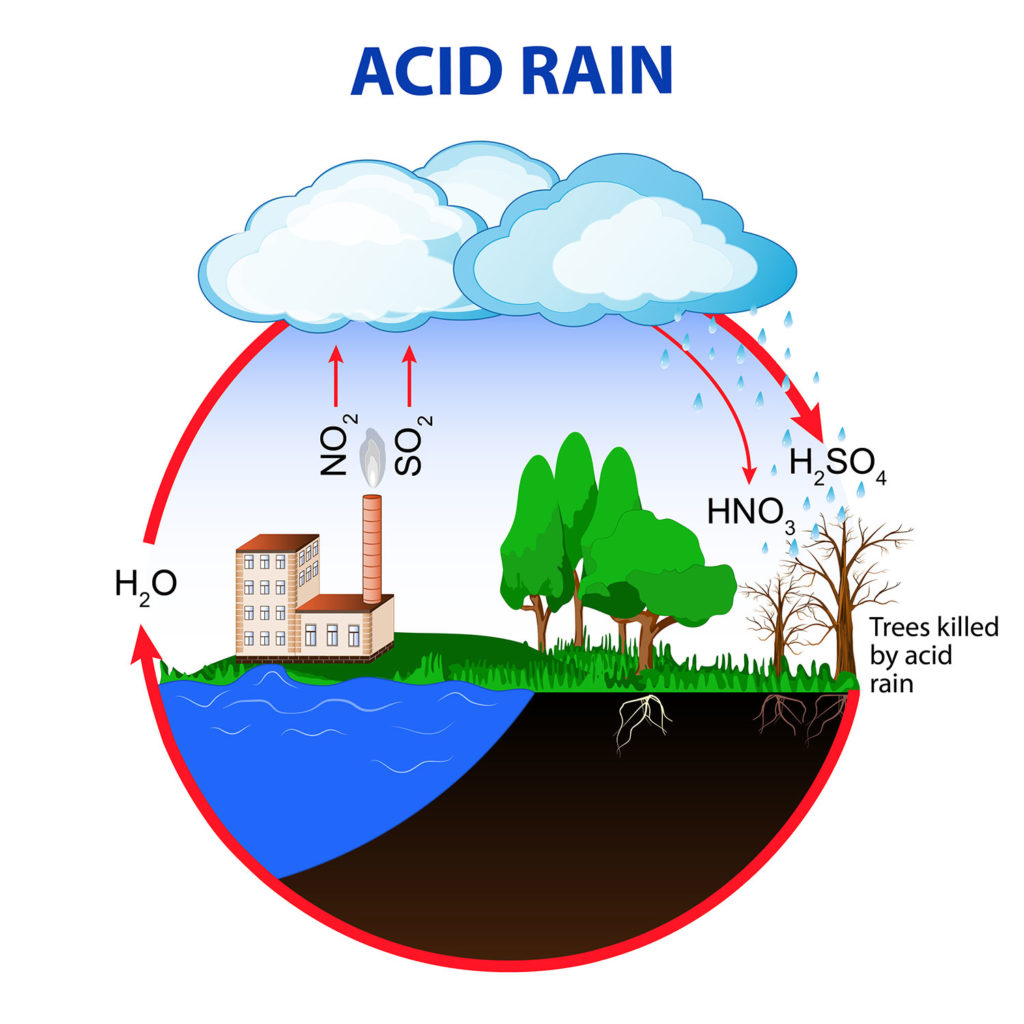
Nitrous oxide and sulfur dioxide emissions, typically from factories and vehicle engines, can combine with water in the atmosphere to form nitric acid and sulfuric acid.
Acid precipitation, also called acid deposition, can be low pH rain, snow, sleet, and even fog.
Acidic precipitation can damage leaves directly, lower the pH of lakes, and erode human-made statues and brickwork.



Check your knowledge. Can you:
-
list examples of different nutrients?
-
provide the sinks and general cycling of water, carbon, and nitrogen?
-
explain how acid rain is formed and describe its effects?


